SleepMed ARES

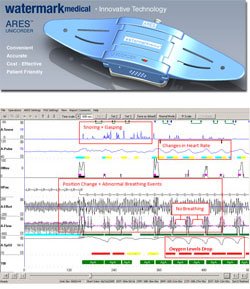
ARES Home Sleep Test A sleep-wearable wireless physiological recorder worn on the forehead that acquires and stores up to 3 nights of nocturnal data. ARES™ measures blood oxygen saturation (SpO2) and pulse rate (reflectance pulse oximetry), airflow (by nasal cannula connected to a pressure transducer), snoring levels (calibrated acoustic microphone), head movement and head position (accelerometers). When worn in the home, the ARES™ provides a better profile of the patient’s breathing during sleep in his/her normal environment. Audio and visual indicators notify the user when the ARES™ requires adjustment, thus increasing the reliability of the device in the home. The small size of ARES™ allows it to be comfortably worn in all sleep position.